Cure of thermosetting polymers - A162
| Cure of thermosetting polymers | |
|---|---|
| Foundational knowledge article | |
| |
| Document Type | Article |
| Document Identifier | 162 |
| Themes | |
| Relevant Class |
Material |
| Tags | |
| Prerequisites | |
Introduction[edit | edit source]
For thermosetting polymers, the manufacturing process step of curing is necessary to transform the viscous polymer resin into a rigid solid. During this process, chemical reactions take place that result in the formation of molecular bonds that set the polymer into shape.
Scope[edit | edit source]
This page provides an overview of the curing process for thermosetting polymers. It covers a brief overview of the chemical curing reaction, physical changes experienced during the curing process, and measurement and monitoring techniques.
Significance[edit | edit source]
The curing process for thermoset polymers is responsible for their polymerization and the formation of the molecular crosslink network of bonds that give thermosets their desirable mechanical properties. This process solidifies the polymer matrix, thereby locking the composite part into its desired shape. Completion of the curing process (complete chemical reaction) is critical for obtaining the full mechanical properties of the polymer.
Prerequisites[edit | edit source]
Recommended documents to review before, or in parallel with this document:
Overview[edit | edit source]
Thermoset polymers are synthesized during the curing process. Polymer chains link together by means of the short crosslink structures, creating a connected rigid network of linked polymer chains. It is this resulting structure that give thermoset polymers their unique mechanical characteristic traits, making thermosets such as epoxy or polyester desirable for use as the matrix component in composite materials.
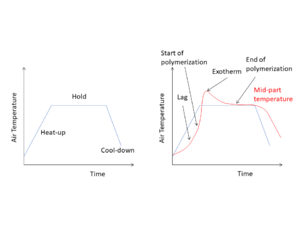
In the curing process, the thermoset polymer transforms from a flowing liquid resin into a rigid polymer. The timing and control of the curing process is a critical process step within the composite manufacturing process, as once the polymer takes on its rigid form, the composite part shape is locked into place and cannot be reshaped. During this transformation process, the thermoset polymer undergoes a series of chemical reactions and physical changes. Full completion of the chemical reactions is necessary for the polymer to acquire its desired properties, particularly its mechanical properties.
For some thermoset polymers, the curing reaction can occur to completion at room temperature, while many other thermoset polymers require a substantial amount of energy in the form of external heat input (e.g. oven or press process) to initiate and complete the process. An initiator (catalyst) may also be involved to initiate and accelerate the polymerization reactions.
Chemical Reaction (Polymerization)[edit | edit source]
During the curing process, the thermoset polymer undergoes polymerization and transitions from a flowing liquid resin into a rigid polymer.
Two polymerization reactions are taking place in this process:
- Linear polymer chains form and grow with the joining together of short monomers
- Crosslinks form between the linear polymer chains
With passing reaction time, this occurs throughout the polymer forming a linked rigid network of polymer molecules. The specific chemical reactions that take place are dependent on the thermoset polymer system resin and hardener.
For some specific examples of these reactions, please see:
Polymer Chain Growth[edit | edit source]
The initial resin state prior to curing consists of short unreacted monomers. These are short repeating molecule units that when reacted together form longer linear polymer chains. This occurs in a variety of methods depending on the thermoset polymer. It can happen by homopolymerization with the input of heat, or through addition reactions where polymer chains are formed by joining the resin monomers to the curing agent molecule to form a co-polymer chain.
The short monomer lengths prior to curing provides the thermoset polymer resin with a low viscosity state as a liquid at room temperature, or at only moderately elevated temperatures (e.g. 100°C). This allows for relative ease of processing into composite materials when compared to thermoplastics [1]. The longer polymer chain length present with thermoplastics, requires high temperature processing for resin flow and processing into composite materials.
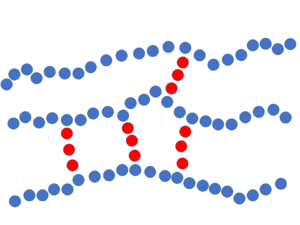
Crosslink Reaction[edit | edit source]
Thermoset polymers used in composites are most often are synthesized by a chemical reaction between two substances: resin and the hardener (crosslinking agent). In these cases, crosslinks between the resin polymer chains are created by addition reaction of the crosslinking agent. The crosslinking agent itself is a short molecule that has reactive sites that react and covalently bond to reactive sites on adjacent polymer chains. In a simple analogy, these short crosslinks bridge the linear polymer chains together. As the crosslink reaction continues, an interconnected three-dimensional network of linked polymer chains is formed that give the thermoset polymer its rigidity.
The frequency of the crosslinks along the linear polymer chain is known as the crosslink density. Increased crosslink density provides the thermoset polymer added rigidity, higher temperature stability (through a higher glass transition temperature), improved resistance to chemical attack, but with increased brittleness.
Physical Changes During the Curing Process[edit | edit source]
During the curing process as polymerization and crosslinking formation takes place, the thermoset polymer goes through several distinct transformation phases.
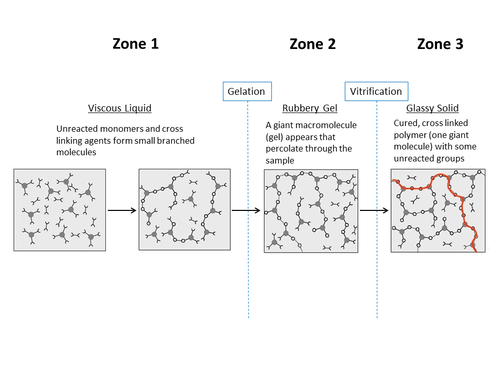
Behavioural stages of a thermoset polymer as it cures:
- Initially the polymer is a flowing viscous liquid
- At gelation, the polymer changes from a viscous liquid to a rubbery gel
- As the curing advances, the rubbery gel transforms into glassy solid at vitrification
Zone 1 - Viscous Liquid Zone, before gelation[edit | edit source]
At room temperature, the resin is highly viscous. As the temperature is increased, the viscosity drops drastically. The reduction of viscosity with temperature continues until curing starts due to the external heat, which causes an increase in molecular weight and thereby viscosity. The viscosity increases rapidly with increasing degree of cure until the resin gels and the resin effectively transforms from a liquid to a solid.
Zone 2 - Rubbery Gel Zone, after gelation and before vitrification[edit | edit source]
As the curing process advances with time and temperature, at a certain degree of cure, a giant macromolecule is formed which spans the material and the material gels. For typical epoxies, gelation occurs at a degree of cure of 0.5 to 0.6. At gelation, the viscosity increases from a finite value to infinity and an insoluble macromolecule (gel) appears in the system. The resin now starts to develop modulus and exhibit viscoelastic properties. As the degree of cure advances, modulus is increased and viscoelastic properties evolved. During this stage, with the advancement of polymerization, the relaxation time and storage modulus of the material increases while loss modulus of the material decreases.
Zone 3 - Glass Solid Zone, after vitrification[edit | edit source]
As the cure advances, the glass transition temperature also advances. Further curing also reduces the free volume in the material (cure shrinkage) and hence the polymer chain mobility. Over a critical free volume range, the chain mobility is hindered and the polymer transforms from a rubbery to a glassy state. This transition, does not occur at a specific temperature but over a temperature range. Glass transition (Tg) + 25℃ is usually mentioned in the literature as the threshold for this transition. Past this transition to the glassy state, due to the low chain mobility, the curing rate drops significantly and consequently modulus development slows down. As a simplification, the material in this stage is usually assumed to behave like an elastic material because the relaxation times are sufficiently long. Most resins never achieve a degree of cure of one (i.e. DOC =1). The final degree of cure is generally 0.85-0.95 for most commercial epoxy systems.
Measuring and Monitoring Cure[edit | edit source]
The crosslinking reaction is exothermic (heat releasing) allowing it to be measured in the laboratory using different methods at various degrees of accuracy to indirectly assess the curing progress. Numerically, the extent of the curing reaction that has occurred in a thermoset polymer can be represented by the degree of cure (DOC) index. It indicates what percentage of the crosslinking reaction has taken place in the polymer.
Other methods that may be used to monitor curing, depending on the thermoset system include:
- Rheological methods
- Dielectric methods
- Spectroscopy (e.g. FTIR) methods
Related pages
References
- ↑ [Ref] Hoa, S V (2018). Principles of the Manufacturing of Composite Materials. DEStech Publications, Incorporated. ISBN 9781605954219.CS1 maint: uses authors parameter (link) CS1 maint: date and year (link)
Welcome
Welcome to the CKN Knowledge in Practice Centre (KPC). The KPC is a resource for learning and applying scientific knowledge to the practice of composites manufacturing. As you navigate around the KPC, refer back to the information on this right-hand pane as a resource for understanding the intricacies of composites processing and why the KPC is laid out in the way that it is. The following video explains the KPC approach:
Understanding Composites Processing
The Knowledge in Practice Centre (KPC) is centered around a structured method of thinking about composite material manufacturing. From the top down, the heirarchy consists of:
- The factory
- Factory cells and/or the factory layout
- Process steps (embodied in the factory process flow) consisting of:
The way that the material, shape, tooling & consumables and equipment (abbreviated as MSTE) interact with each other during a process step is critical to the outcome of the manufacturing step, and ultimately critical to the quality of the finished part. The interactions between MSTE during a process step can be numerous and complex, but the Knowledge in Practice Centre aims to make you aware of these interactions, understand how one parameter affects another, and understand how to analyze the problem using a systems based approach. Using this approach, the factory can then be developed with a complete understanding and control of all interactions.
Interrelationship of Function, Shape, Material & Process
Design for manufacturing is critical to ensuring the producibility of a part. Trouble arises when it is considered too late or not at all in the design process. Conversely, process design (controlling the interactions between shape, material, tooling & consumables and equipment to achieve a desired outcome) must always consider the shape and material of the part. Ashby has developed and popularized the approach linking design (function) to the choice of material and shape, which influence the process selected and vice versa, as shown below:
Within the Knowledge in Practice Centre the same methodology is applied but the process is more fully defined by also explicitly calling out the equipment and tooling & consumables. Note that in common usage, a process which consists of many steps can be arbitrarily defined by just one step, e.g. "spray-up". Though convenient, this can be misleading.
Workflows
The KPC's Practice and Case Study volumes consist of three types of workflows:
- Development - Analyzing the interactions between MSTE in the process steps to make decisions on processing parameters and understanding how the process steps and factory cells fit within the factory.
- Troubleshooting - Guiding you to possible causes of processing issues affecting either cost, rate or quality and directing you to the most appropriate development workflow to improve the process
- Optimization - An expansion on the development workflows where a larger number of options are considered to achieve the best mixture of cost, rate & quality for your application.
To use this website, you must agree to our Terms and Conditions and Privacy Policy.
By clicking "I Accept" below, you confirm that you have read, understood, and accepted our Terms and Conditions and Privacy Policy.