Thermal phase transitions of polymers - A102
| Thermal phase transitions of polymers | |
|---|---|
| Foundational knowledge article | |

| |
| Document Type | Article |
| Document Identifier | 102 |
| Themes | |
| Relevant Class |
Material |
| Tags | |
| Prerequisites | |
Introduction[edit | edit source]
Polymers go through several distinct phase transition points at particular temperatures. These transitions induce changes to their specific volume, mechanical properties, and physical behaviour.
The most significant thermal transition points include melting (thermoplastics only), and glass transition temperature.
Scope[edit | edit source]
This page provides a brief overview to the temperature dependent phase transitions experienced by polymers upon heating and cooling. The key temperature points: melting temperature (Tm) and glass transition temperature (Tg) are discussed for their physical significance.
Significance[edit | edit source]
Upon heating and cooling, polymers experience distinct phase transitions when passing through specific temperatures. When temperature is raised, they physically transition from solid-like glassy to rubbery, and in the case of thermoplastics from rubbery to liquid like flow with additional heating. These transitions take place in reverse when cooling.
The temperature points where these phase transitions occur are unique to each specific polymer. Awareness of these key temperature points is important as it has direct implications to both the processing and in-service use of the polymer.
Prerequisites[edit | edit source]
Recommended documents to review before, or in parallel with this document:
Overview[edit | edit source]
Polymers go through several distinct phase transition points at particular temperatures. These transitions induce changes to their specific volume, mechanical properties, and physical state.
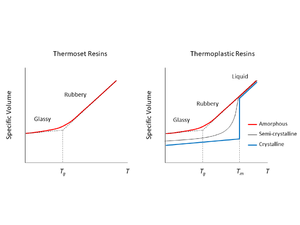
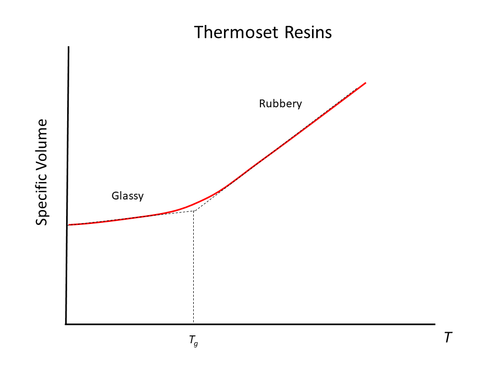
The most significant thermal transition points include melting (thermoplastics only), and glass transition temperature. The following figure illustrates these key transition points for thermoset and thermoplastic polymers:
Key Temperature Points[edit | edit source]
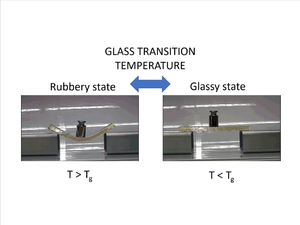
Glass transition temperature (Tg) – The temperature region where the polymer behaviour transitions from a hard-glassy material, to that of a soft rubbery material. A sudden loss in mechanical stiffness occurs.
Melting temperature (Tm) – The temperature point at which the polymer physically transitions from a solid to viscous flow upon heating, or vice versa – from melt to a solid upon cooling.
Whether or not a polymer exhibits all of the illustrated transitions and whether they occur at a discrete temperature point or a temperature range is dependent on the polymer’s molecular structure.
Behavior Regions[edit | edit source]
Glassy – The polymer behaves as a stiff, elastic solid. Polymer chains are locked in their configuration with no mobility to re-orient themselves.
Rubbery – Polymer softens and gains flexibility. It behaves as a viscoelastic material. Some mobility of the polymer chains is gained in amorphous regions.
Liquid Melt – The polymer behaves with liquid-like fluid motion (viscous material). Polymer chains loosen from their crumpled configurations and are able to move freely, re-orienting in configuration with each other.
Melting Temperature (Tm)[edit | edit source]
Link to main Melting Temperature page
The phase transition of melting is a characteristic exhibited by thermoplastics. For thermosets, melt and subsequent flow is not physically possible due to their fundamental molecular crosslink structure.
Heated beyond the melting temperature (Tm), the polymer transitions from a solid to a viscous liquid like behaviour that can flow. At this phase change, a polymer experiences a large change in specific volume and the polymer chains gain mobility. In reverse, the process of cooling from above Tm, dropping below it to solidify, is referred to as crystallization.
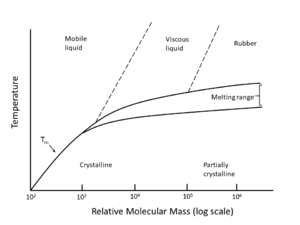
For low relative molecular mass (RMM), 103 and below, Tm occurs at a distinct temperature point. At higher RMM, melting occurs over a broadened range of temperature [1]. In reality, even for low RMM and linear polymers, the melting transition is generally observed to take place over a temperature range rather than at a distinct temperature point due to the variation in crystal sizes present in the polymer.
Crystallization[edit | edit source]
Link to main Melt and Crystallization of Thermoplastic Polymers page
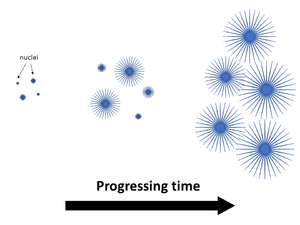
Upon cooling, polymer chains begin the process of re-organizing themselves from random orientations into packed folded structures of some periodic order (crystals). The crystallization process initiates as nuclei points in the melt. A common crystalline structure formed are spherulites (as pictured).
As cooling continues, the chain folded crystalline structure regions continue to grow in area, while areas of random chain orientation without order are considered amorphous. The term semi-crystalline denotes a polymer consisting of both crystalline and amorphous regions. In the pictured spherulite illustration, the areas between the spherulite crystal structures are amorphous.
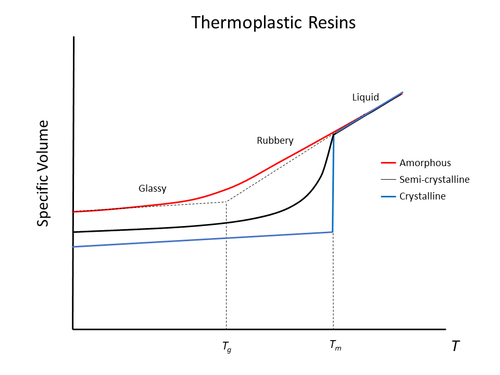
For thermoplastics, the transition from glassy to liquid melt is affected by the proportion of crystallinity in the polymer. Specifically, the rubbery transition range is heavily influenced by crystallinity due to the need to unpack and unravel the crystal structures for fluid-like motion to occur.
The greater the crystallinity, the less rubbery behaviour that is observed in transitioning from glassy to a liquid melt.
Click here to learn more about melt and crystallization of thermoplastic polymers.
Glass Transition Temperature (Tg)[edit | edit source]
Link to main Glass Transition Temperature page
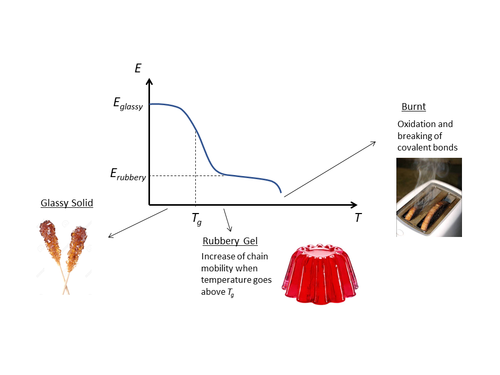
Polymers are classified as glassy when their molecular backbones exhibit the inability to move and remain “frozen” in crumpled immobile conformations. Below Tg, upon heating only thermally induced expansion between the molecules occurs. Above Tg, amorphous regions within the polymer observe liquid-like “flow” with the molecular chains gaining rapid ability to move freely, while crystalline regions remain locked in the glassy state configuration.
As an outcome of this behavioral transition, a polymer exhibits a sudden change in the response of specific volume to change in temperature (thermal expansion) at the Tg point. Mechanically, a softening drop in mechanical stiffness occurs. This substantial drop in mechanical stiffness is shown in the example picture, showing a polymer below and above its glass transition temperature.
At temperatures above Tg, a substantial reduction in Young’s Modulus (E) is observed between the stiff glassy state and the softened rubbery state. To ensure in service mechanical stiffness of the polymer, the operational temperature should be below Tg. However, there are situations where having the service temperature above the polymer’s Tg to obtain rubbery behaviour is intended. Elastomers are the classic example, where their rubbery behaviour is desired and the material is used above its Tg.
Click here to learn more about glass transition temperature.
Related pages
| Page type | Links |
|---|---|
| Introduction to Composites Articles | |
| Foundational Knowledge Articles | |
| Foundational Knowledge Method Documents | |
| Foundational Knowledge Worked Examples | |
| Systems Knowledge Articles | |
| Systems Knowledge Method Documents | |
| Systems Knowledge Worked Examples | |
| Systems Catalogue Articles | |
| Systems Catalogue Objects – Material | |
| Systems Catalogue Objects – Shape | |
| Systems Catalogue Objects – Tooling and consumables | |
| Systems Catalogue Objects – Equipment | |
| Practice Documents | |
| Case Studies | |
| Perspectives Articles |
|
References
| About | Help |
Welcome
Welcome to the CKN Knowledge in Practice Centre (KPC). The KPC is a resource for learning and applying scientific knowledge to the practice of composites manufacturing. As you navigate around the KPC, refer back to the information on this right-hand pane as a resource for understanding the intricacies of composites processing and why the KPC is laid out in the way that it is. The following video explains the KPC approach:
Understanding Composites Processing
The Knowledge in Practice Centre (KPC) is centered around a structured method of thinking about composite material manufacturing. From the top down, the heirarchy consists of:
- The factory
- Factory cells and/or the factory layout
- Process steps (embodied in the factory process flow) consisting of:
The way that the material, shape, tooling & consumables and equipment (abbreviated as MSTE) interact with each other during a process step is critical to the outcome of the manufacturing step, and ultimately critical to the quality of the finished part. The interactions between MSTE during a process step can be numerous and complex, but the Knowledge in Practice Centre aims to make you aware of these interactions, understand how one parameter affects another, and understand how to analyze the problem using a systems based approach. Using this approach, the factory can then be developed with a complete understanding and control of all interactions.
Interrelationship of Function, Shape, Material & Process
Design for manufacturing is critical to ensuring the producibility of a part. Trouble arises when it is considered too late or not at all in the design process. Conversely, process design (controlling the interactions between shape, material, tooling & consumables and equipment to achieve a desired outcome) must always consider the shape and material of the part. Ashby has developed and popularized the approach linking design (function) to the choice of material and shape, which influence the process selected and vice versa, as shown below:
Within the Knowledge in Practice Centre the same methodology is applied but the process is more fully defined by also explicitly calling out the equipment and tooling & consumables. Note that in common usage, a process which consists of many steps can be arbitrarily defined by just one step, e.g. "spray-up". Though convenient, this can be misleading.
Workflows
The KPC's Practice and Case Study volumes consist of three types of workflows:
- Development - Analyzing the interactions between MSTE in the process steps to make decisions on processing parameters and understanding how the process steps and factory cells fit within the factory.
- Troubleshooting - Guiding you to possible causes of processing issues affecting either cost, rate or quality and directing you to the most appropriate development workflow to improve the process
- Optimization - An expansion on the development workflows where a larger number of options are considered to achieve the best mixture of cost, rate & quality for your application.
To use this website, you must agree to our Terms and Conditions and Privacy Policy.
By clicking "I Accept" below, you confirm that you have read, understood, and accepted our Terms and Conditions and Privacy Policy.