Effect of material in a thermal management system - A155
| Effect of material in a thermal management system | |
|---|---|
| Systems knowledge article | |
| Document Type | Article |
| Document Identifier | 155 |
| Themes | |
| Relevant Classes |
|
| Tags | |
| Prerequisites | |
Introduction[edit | edit source]
Perhaps the most obvious system parameter is the part material. A part is fully defined by the definition of material(s) and shape. From a thermal management perspective, a material is defined through properties such as density, thermal conductivity, specific heat capacity, and cure kinetics (or melt/ crystallization kinetics). A change in the material system will alter these parameters and will, in turn, alter the thermal response of the part. It is not just what happens in the thermal transformation (cure) cell/step that matters. The thermal history of the material throughout the manufacturing lifecycle plays a huge role in the outcome of the part. For example, if the material was not stored at the proper temperature, or if the initial temperature is not as expected during the cure/thermal transformation step, then the part's final degree of cure, and other physical and mechanical properties may be impacted.
Scope[edit | edit source]
This article discusses the impact the material has on the thermal response of the part. The effect of various material parameters are investigated from a thermal management perspective. The page links out to foundational knowledge content and brings in physics-based simulation to demonstrate the impact of the material in a thermal system. While the content presented here is applicable to thermal management in all factory cells, the focus of this page is on the thermal transformation cell as that is the predominant cell associated with thermal management. This article is intended for SMEs and other interested parties who may be looking at a variety of material systems from a thermal perspective. It does not provide an in-depth investigation into the differences in thermal properties between different material manufacturers. To understand the specifics of a given material system, it is best to contact the manufacturer.
Significance[edit | edit source]
Thermal management of raw material begins from the moment they are shipped from the supplier through to thermal transformation/cure. During this time, the temperature of the material (referred to as its temperature history), matters greatly and can affect the final mechanical properties of the part. Because of this, almost all material systems will have thermal specifications from the manufacturer. This may include storage temperature, out-time, curing temperature, temperature ramp rate, cooling rate, etc. For companies in the supply chain of a larger OEM, the material system is likely predefined and may even be constrained from a thermal perspective. That is, a practitioner may not have the freedom to change aspects of their process such as the maximum applied temperature or temperature ramp rate. However, other companies may not be subject to such constraints and even have the freedom to select their material system and process requirements. Small changes in the material system may have dramatic effects on the material's thermal properties and, therefore, the part's thermal response. An understanding of such effects and how they come together with the part shape, tooling, and equipment parameters is highly important.
Prerequisites[edit | edit source]
Recommended documents to review before, or in parallel with this document:
- General material properties
- Polymer properties
- Reinforcement properties
- Composite properties
- Heat transfer
- Thermal management
- System interactions
- Material (system class)
Overview[edit | edit source]
The material defines the thermal properties of the part. This includes density, thermal conductivity, specific heat capacity, thermal diffusivity, fibre volume fraction, and any cure or crystallization properties (heat of reaction, glass transition temperature, melt temperature, etc.). Taking into account the part shape and mass (influenced by the part thickness), these properties control the part's thermal response. Changing the material system may drastically influence these properties, and thus the thermal response of the part.
By defining the part material and shape, the outcome sensitivity of the system is also defined. That is, how the outcomes will be influenced by the imposed boundary conditions. In other words, defining the material and shape locks in the thermal behaviour of the part. At this point, in order to change the part outcomes, only the boundary conditions themselves can be changed. This can be done by altering parameters of the equipment or tooling.
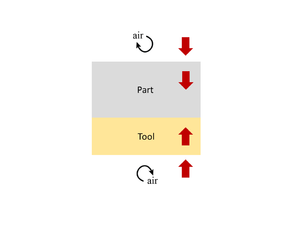
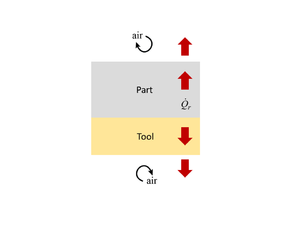
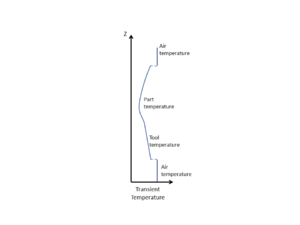
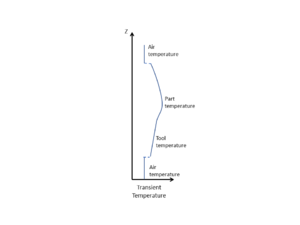
When conducting a thermal analysis of the system, it can often be assumed that heat transfer occurs in 1D, through the thickness of the material (both part and tool). The reason for this is that in-plane dimensions are typically much larger than the thickness dimension. Even allowing for the fact that fibres can be more thermally conductive than resin, the in-plane heat transfer, and resulting thermal heat gradients, are much smaller as compared with the through-thickness direction. Therefore, the predominant direction of heat transfer is through the material stack, rather than in-plane. This is clearly an approximation, but is typically considered to be a conservative assessment. Where the part dimensions are clearly 2D or 3D in nature (the thickness is not small compared to either of the in-plane dimensions) then a 1D approximation is not valid. The nature of the tooling, particularly if made of highly conductive materials such as aluminum, may also affect a 1D approximation validity.
| Heat-up | Exotherm |
|---|---|
Heat transfer into and out of the part occurs by conduction through the tool-part assembly. If the temperature across the part surfaces (or tooling surfaces) is known, then the temperature within the part can be approximated using the 1D form of the heat balance equation, as shown below.
Where,
To perform a thermal profile of a tool/part, refer to the following pages:
Material type[edit | edit source]
Generally speaking, the part material consists of the composite (matrix and reinforcement) and any inserts (such as core materials). Ignoring tougheners or other additives, composite materials can be broken into families based on the matrix and/or reinforcement. For the purpose of this page, only fibre reinforcements and thermosetting resin matrices will be considered. There are many families of fibres and thermosetting resins used in polymer-composites. Below are perhaps the most commonly used:
Fibre families:
Thermosetting resin families:
While any combination of fibre and resin is possible, typically carbon fibres are used in combination with epoxies, while glass is often used in combination with vinyl esters and polyesters. Aramid is commonly found with epoxies, polyesters, or other resins such as phenolic resins. From a thermal management perspective, the resin controls the cure or crystallization properties of the composite, while the resin and fibres determine the density, thermal conductivity, specific heat capacity, and other thermal properties of the part. All of these properties are influenced by the fibre volume fraction, which also dictates the amount of resin present. Furthermore, depending on the raw material form or the manner in which its processed, the fibre volume fraction and other thermal properties may change. For example, unidirectional composites have a higher fibre volume fraction than fabrics and therefore a lower amount of resin. Similarly, prepregs allow for a higher fibre volume fraction as compared with processes involving deposition of resin and fibre individually. Finally, debulking and thermal transformation influence the fibre volume fraction and other material properties by void removal (or growth), resin flow, and laminate consolidation.
The energy required to heat a part is determined by its thermal mass, as governed by the following equation:
\(Q=mC_p\Delta{T}\)Energy required to raise an object's temperature, where \(Q\) = energy, \(m\) = object mass, \(C_p\) = specific heat capacity, \(\Delta{T}\) = change in temperature from applied energy. Note that \(mC_p\) is the thermal mass of the object.
The efficiency in which heat transfers through the part is determined by the thermal diffusivity, calculated using the following equation:
\(\alpha=\frac{k}{\rho C_p}\)Thermal diffusivity, where \(\alpha\) = thermal diffusivity, \(k\) = thermal conductivity, \(\rho\) = density, and \(C_p\) = specific heat capacity.
Changing the fibre, resin, material form, or processing conditions, may have an effect on the thermal response of the part, due to a change in the material properties of the composite. A carbon-epoxy will behave much differently than a glass-polyester and may require different processing conditions in order to achieve the nominal mechanical properties. Generally speaking, a change in resin is a much more significant change from a thermal management perspective than a change in the fibre type. However, both may have notable affects on the part's thermal response and should be considered. While changing the resin or fibre family may seem obvious that it will have an effect on the part's thermal response, even changing the specific type of material within the same family may have notable effects. This is particularly true for the resin and can be observed in a number of epoxies, where some require processing at high temperature, yet others can be cured at room temperature. Even amongst epoxies (and other resins) where the curing temperatures are similar, there can be variations in the material response due to changes in material properties.
Epoxy resin[edit | edit source]
| Unidirectional carbon-epoxy A | Unidirectional carbon-epoxy B | Unidirectional glass-epoxy A |
|---|---|---|
The above figure shows two different high temperature, carbon-epoxy parts and one glass-epoxy part. Note that the heat transfer coefficient (HTC) is 20 W/m2K to represent curing in an oven. While both carbon-epoxies exhibit similar thermal responses, there is a slight difference in their maximum part temperature and degree of cure. Carbon-epoxy A reaches a higher temperature upon exotherm, yet achieves a lower final DOC as compared with epoxy B. Typically, one would expect a higher max temperature to result in a higher DOC. This goes to show that even similar material systems do not necessarily yield the same outcomes even if the thermal response is similar. The glass-epoxy system uses the same resin as carbon-epoxy A, and with the same fibre volume fraction, but with a change in fibre type. This doesn't have significant effects on the thermal response of the part, but there are some differences. The thermal lag increases slightly while the maximum temperature decreases slightly. This is likely due to the increase in thermal mass (increase in fibre density and specific heat capacity), resulting in a longer heat up time and higher capacity for energy absorption. It should also be noted that the diffusivity of glass-epoxy A is less than that of carbon-epoxy A, which could result in a more uneven temperature distribution across the part.
Material properties for the three composites are provided in the table below.
| Material property | Epoxy A | Epoxy B | Carbon fibre (same properties for both carbon composites) | Glass fibre (E-glass) | Carbon-epoxy A (composite A) | Carbon-epoxy B (composite B) | Glass-epoxy A (composite C) |
|---|---|---|---|---|---|---|---|
| Heat of reaction (J/kg) | 540,000 | 437,000 | NA | NA | NA | NA | NA |
| Resin density (Kg/m3) | 1300 | 1180 | NA | NA | NA | NA | NA |
| Resin degradation temperature (°C) | 190 | 200 | NA | NA | NA | NA | NA |
| Resin specific heat capacity (J/Kg\(\cdot\)K) | 1005 | 1196 | NA | NA | NA | NA | NA |
| Resin thermal conductivity (W/m\(\cdot\)K) | 0.148 | 0.20 | NA | NA | NA | NA | NA |
| Fibre density (Kg/m3) | NA | NA | 1760 | 2560 | NA | NA | NA |
| Fibre specific heat capacity (J/Kg\(\cdot\)K) | NA | NA | 750 | 806 | NA | NA | NA |
| Fibre thermal conductivity (W/m\(\cdot\)K) | NA | NA | 7.69 (longitudinal), 2.4 (transverse) | 1.28 longitudinal and transverse | NA | NA | NA |
| Fibre volume fraction | NA | NA | NA | NA | 0.57 | 0.59 | 0.57 |
Between the carbon composites, the first parameter to note is the difference in heat of reaction. Epoxy A has a 24% larger heat of reaction than epoxy B. Moreover, composite A has a slightly lower fibre volume fraction (and therefore higher resin volume fraction) and a higher resin density than composite B. This explains why composite A undergoes a larger exotherm. However, due to the kinetics of its cure reaction it achieves a lower DOC in the end. This means that not all polymer chains have cross-linked and the material does not realize the full potential of its exothermic heat output. If a higher temperature was applied, it is likely the material would continue to output heat and reach a higher DOC. Though, it should be noted that under the imposed conditions, epoxy A already goes above its degradation temperature (190°C) while epoxy B remain close but below its degradation temperature of 200°C. Therefore, the 0.84 final DOC that composite A achieves may not be indicative of the true mechanical properties, since the material has likely degraded somewhat. Even for epoxy B, although the degradation temperature was found to be 200°C, the maximum processing temperature for both resins, as recommended by the manufacturers is 180°C. Therefore, even for composite B it's not ideal that it reaches the 190°C that is shown in the figure.
Note that the thermal conductivity of the carbon fibre is significantly higher than either of the resins, both in the fibre (longitudinal) and transverse direction. Glass fibres are generally less thermally conductive than carbon fibres, as is the case here. However, the E-glass fibre is still much more thermally conductive than the epoxy resin. In some cases, the glass fibre type (or other fibre type) may have a thermal conductivity comparable with the resin. Generally speaking, the fibres themselves are only in-plane (i.e. not through the thickness of the material), unless a 3D fibre architecture is implemented (such as a 3D braid for example).
Polyester resin[edit | edit source]
All else equal, a change in reinforcement family (eg. from carbon to glass fibre) won't typically result in a drastically different thermal response (as shown above). However, a change in resin family (eg. from epoxy to polyester) will have a large impact on the thermal response of the part. Polyesters, cure at room temperature, using their exothermic heat to advance the cure reaction. Most epoxies used for industrial applications cure at high temperatures, utilizing externally applied heat and, to a lesser extent, their heat of reaction to advance their cure. Moreover, the chemical formulation of polyesters can be, and is often, changed in order to attain the appropriate cure behaviour. This involves changing the initiator concentration as well as including or altering the concentration of a number of chemical additives. This can have a significant impact on the cure behaviour and properties of the polyester. As shown above, while not all high-temperature epoxies have the same thermal response, this is even more true for polyesters, as their chemical formulation may vary widely. In contrast, the chemical formulation of high-temperature epoxies is typically not altered or the alterations are relatively minor in comparison.
A typical cure reaction curve for a glass-polyester composite cured at room temperature is provided below. Notice how the part temperature is almost entirely driven by the heat of reaction of the resin. Also note that the HTC is 5 W/m2K to represent curing in stagnant air.
| Glass-polyester A |
|---|
Material properties for the glass-polyester composite are provided in the table below. The glass fibre is the same as that shown in the previous table/figures.
| Material property | Polyester A | Glass fibre (E-glass) | Glass-polyester A (composite D) |
|---|---|---|---|
| Heat of reaction (J/kg) | 311,000 | NA | NA |
| Resin density (Kg/m3) | 1400 | NA | NA |
| Resin specific heat capacity (J/Kg\(\cdot\)K) | 1162 | NA | NA |
| Resin thermal conductivity (W/m\(\cdot\)K) | 0.20 | NA | NA |
| Fibre density (Kg/m3) | NA | 2560 | NA |
| Fibre specific heat capacity (J/Kg\(\cdot\)K) | NA | 806 | NA |
| Fibre thermal conductivity (W/m\(\cdot\)K) | NA | 1.28 longitudinal and transverse | NA |
| Fibre volume fraction | NA | NA | 0.21 |
Material form[edit | edit source]
As mentioned previously, the material form plays a role in dictating the thermal response of the part. A large reason for this has to do with the fibre volume fraction. Different material forms allow for a higher or lower fibre volume fraction which, in other words, means they allow for a higher or lower resin volume fraction. Aside from altering the thermal properties of the composite according to the rule of mixtures, material forms with a higher resin volume fraction are more susceptible to larger exotherms due to the increase in the weight percent of resin. Consider the figures below. In both cases, the epoxy and fibre are the same but the fibre architecture is different. Namely, in the first case the material is a unidirectional (UD) fibre prepreg whereas in the second case a plain weave (PW) fabric prepreg is used. UD prepregs allow for a higher fibre volume fraction than PW prepregs. What this also means is that UD prepregs have less resin content per volume as compared with a PW prepreg. Therefore, for the same laminate size and processing conditions, a PW prepreg will exhibit a larger exotherm. In this case, the UD prepreg has a fibre volume fraction of 0.57 compared with 0.55 for the PW prepreg, so the difference is not a lot. However, this still allows for a slightly larger exotherm in the PW prepreg, resulting in a maximum temperature increase of 1.3°C as compared with the UD prepreg. In both cases, the maximum temperature is above the material specifications and measured degradation temperature (see the above table) and will, therefore, likely result in thermal degradation of the part.
| Unidirectional carbon with epoxy A | Plain weave carbon with epoxy A |
|---|---|
To learn how to measure fibre volume fraction, visit the following page
Another important distinction from a thermal perspective is whether the part is constructed as a laminate or a sandwich panel. Aside from the altered mechanical performance offered by a sandwich construction, the addition of a core can have significant thermal effects, depending on the core material and thickness. In the figures below, the same carbon-epoxy as presented above is utilized in a laminate and sandwich construction. In the sandwich panel case, the skins are 5mm thick, giving a total composite thickness of 10mm, the same as for the laminate case. Focussing on the central figure, it can be seen that the addition of an aramid core results in a higher maximum temperature for the top skin, but a lower maximum temperature for the bottom skin as compared with the laminate case. This is because the core adds thermal insulation to the part. Therefore, as the top skin begins to exotherm, heat cannot easily flow from this skin, through the part, and into the tool - as typically occurs. As a result, more heat is forced to transfer out of the top skin via the interface with the air. Unless the heat transfer coefficient (HTC) is significantly high, this is not as effective as transferring heat through the tool interface. Therefore, the top skin heats up. The bottom skin on the other hand is adjacent to the aluminum tool which has a high thermal conductivity. As a result, heat can easily flow from the bottom skin into the tool. Because the bottom skin is only 5mm as compared with the 10mm laminate, the total amount of heat that must escape is less, and the maximum temperature of the bottom skin is reduced.
In the third figure, the core material is aluminum rather than aramid. In this case, the material response is very similar to the laminate case. This is because the aluminum core has a high thermal conductivity, significantly higher than aramid. As a result heat can easily flow through the core. In that sense, the core can effectively be ignored and the part behaves similar to as if it were a laminate. However, the temperature is reduced slightly as compared with the laminate case. This is likely due to the fact that the aluminum behaves as a heat sink with respect to the skins, allowing heat to be dissipated over a larger area and improving heat transfer.
It should also be noted, that in both sandwich panel cases, the core itself does not exotherm. Rather it heats up as the top and bottom skins exotherm.
| Laminate: Unidirectional carbon-epoxy A | Sandwich panel: Unidirectional carbon-epoxy A with aramid core | Sandwich panel: Unidirectional carbon-epoxy A with aluminum core |
|---|---|---|
This analysis results presented above may differ significantly depending on the part thickness, tooling material and thickness, and the HTC. To learn more about these effects visit the following pages:
- Effect of shape in a thermal management system
- Effect of tooling in a thermal management system
- Effect of equipment in a thermal management system
Complicating factors/edge cases[edit | edit source]
Material systems are manufacturer dependendent. Small changes in the material formulation may result in drastic changes in the thermal response of the part. Moreover, it may be difficult to gather all the thermochemical and thermophysical properties of a given material system. To understand a material system it is best to refer to the technical data sheet and/or contact the manufacturer. This page is intended only to highlight the general effects that the material plays in a thermal system. Results will differ due to other processing parameters such as part thickness, tooling thickness/material, HTC values, equipment choice, and others. Finally, in the generated curves, consumables were not considered for simplicity. Depending on the consumables used and their thickness, they may have a notable effect on the part response.
Related pages
| Page type | Links |
|---|---|
| Introduction to Composites Articles | |
| Foundational Knowledge Articles | |
| Foundational Knowledge Method Documents | |
| Foundational Knowledge Worked Examples | |
| Systems Knowledge Articles | |
| Systems Knowledge Method Documents | |
| Systems Knowledge Worked Examples | |
| Systems Catalogue Articles | |
| Systems Catalogue Objects – Material | |
| Systems Catalogue Objects – Shape | |
| Systems Catalogue Objects – Tooling and consumables | |
| Systems Catalogue Objects – Equipment | |
| Practice Documents | |
| Case Studies | |
| Perspectives Articles |
References
- ↑ The curves presented on this page were generated using RAVEN software by Convergent Manufacturing Technologies. Other thermal simulation software exists and CKN is not endorsing use of RAVEN over other software packages.
| About | Help |
Welcome
Welcome to the CKN Knowledge in Practice Centre (KPC). The KPC is a resource for learning and applying scientific knowledge to the practice of composites manufacturing. As you navigate around the KPC, refer back to the information on this right-hand pane as a resource for understanding the intricacies of composites processing and why the KPC is laid out in the way that it is. The following video explains the KPC approach:
Understanding Composites Processing
The Knowledge in Practice Centre (KPC) is centered around a structured method of thinking about composite material manufacturing. From the top down, the heirarchy consists of:
- The factory
- Factory cells and/or the factory layout
- Process steps (embodied in the factory process flow) consisting of:
The way that the material, shape, tooling & consumables and equipment (abbreviated as MSTE) interact with each other during a process step is critical to the outcome of the manufacturing step, and ultimately critical to the quality of the finished part. The interactions between MSTE during a process step can be numerous and complex, but the Knowledge in Practice Centre aims to make you aware of these interactions, understand how one parameter affects another, and understand how to analyze the problem using a systems based approach. Using this approach, the factory can then be developed with a complete understanding and control of all interactions.
Interrelationship of Function, Shape, Material & Process
Design for manufacturing is critical to ensuring the producibility of a part. Trouble arises when it is considered too late or not at all in the design process. Conversely, process design (controlling the interactions between shape, material, tooling & consumables and equipment to achieve a desired outcome) must always consider the shape and material of the part. Ashby has developed and popularized the approach linking design (function) to the choice of material and shape, which influence the process selected and vice versa, as shown below:
Within the Knowledge in Practice Centre the same methodology is applied but the process is more fully defined by also explicitly calling out the equipment and tooling & consumables. Note that in common usage, a process which consists of many steps can be arbitrarily defined by just one step, e.g. "spray-up". Though convenient, this can be misleading.
Workflows
The KPC's Practice and Case Study volumes consist of three types of workflows:
- Development - Analyzing the interactions between MSTE in the process steps to make decisions on processing parameters and understanding how the process steps and factory cells fit within the factory.
- Troubleshooting - Guiding you to possible causes of processing issues affecting either cost, rate or quality and directing you to the most appropriate development workflow to improve the process
- Optimization - An expansion on the development workflows where a larger number of options are considered to achieve the best mixture of cost, rate & quality for your application.
To use this website, you must agree to our Terms and Conditions and Privacy Policy.
By clicking "I Accept" below, you confirm that you have read, understood, and accepted our Terms and Conditions and Privacy Policy.