Differential scanning calorimetry (DSC) - A192
Introduction[edit | edit source]
A Differential Scanning Calorimeter (DSC) measures the heat flow in and out of a sample relative to a reference during a predefined thermal cycle. This test provides quantitative and qualitative insights into physical and chemical transformations during endothermic and exothermic processes. The samples are placed in a controlled temperature environment alongside the reference pan (see Figure 1). Thermocouples embedded in the test chamber allow temperature to be recorded with time. The temperature of the test chamber is controlled by cooling accessories and heating elements surrounding the chamber. The electrical power consumed by the equipment to maintain the predetermined thermal cycle is recorded and represented to the user as Heat Flow.
In many cases, the linear temperature ramps in dynamic tests are combined with sinusoidal heating profiles such that while the sample experiences an average increase in temperature, it undergoes instantaneous heating and cooling. This modulation is sufficient to determine the glass transition temperature.
While a DSC provides detailed reliable data on cure kinetics for thermosetting polymers, it is important to not exceed the temperature beyond the degradation temperature. Hence, oftentimes, an extra sample will be prepared for testing in the Thermogravimetric Analyzer (TGA) before beginning the series of DSC tests.
Features[edit | edit source]
The DSC environmental chamber is designed to measure properties like glass transition temperature, crystallization, phase changes, product stability, oxidative stability, cure and cure kinetics among others. By comparing against an empty 'reference' pan, the DSC is able to log heat flow into and out of the pan containing the sample with high sensitivity while temperature in the chamber is controlled.
Cooling Accessories[edit | edit source]
Different cooling accessories exist that allow different temperature ranges to be scanned. For example, Refrigerated Cooling System (RCS) and Discovery Liquid Nitrogen Pump Accessory (LN2P) are provided by TA Instruments (look at TA Instruments Brochure), which can achieve temperatures as low as -90 °C and -120 °C, respectively.
Auto-Samplers[edit | edit source]
Some DSCs are equipped with auto-samplers that allow for tests to be run sequentially. This consists of a built-in XYZ gantry system and gripper to move samples from a tray to the test chamber. Grippers can be sensitive to dimensional changes of the pans so care must be taken to prevent any damage during sample preparation.
Other[edit | edit source]
Several other accessories are available to allow the collection of more qualitative data. Some examples include a microscope camera that can be used to take pictures of the sample as it experiences thermal events. Other optical accessories can be used to capture the optical characteristics of samples.
Uses and test types[edit | edit source]
As noted previously, a DSC is used to quantitatively determine the heat energy absorbed/released by the sample, thereby allowing the computation of DOC (non-reversible heat flow) and Tg (reversible heat flow – corresponds to specific heat capacity of the sample). It does this through three types of tests.
Dynamic Tests[edit | edit source]
Dynamic tests are the default test type used in DSC. The sample undergoes a linear temperature ramp during heating and cooling, producing a heat flow vs temperature graph. Tm, Tc, and Tg are some properties that can be determined from this test.
Isothermal Tests[edit | edit source]
An isothermal test is simply a temperature hold. Temperature holds are frequently added to dynamic tests to allow the material to reach thermal equilibrium before the next temperature ramp. Isothermal tests can also be valuable for analyzing thermoset polymers (Thermoset polymers). The thermoset sample undergoes the cure reaction (Cure of thermosetting polymers) while exposed to a constant temperature for extended periods of time. The heat released by the cure reaction, reaction rate, and total cure time can be determined from this procedure. These tests more accurately represent the cure cycles used by industry.
Modulated DSC Tests[edit | edit source]
MDSC tests include a sinusoidal heating profile superimposed on a temperature ramp with constant rate. This allows properties that would normally be masked by other effects (such as Tg) to be more accurately defined. For example, in a regular DSC test, thermal events frequently occur during the same temperature range which can sometimes mask weaker events such as the glass transition. MDSC allows the data to be analyzed separately by separating total measured heat flow into reversing and non-reversing heat flow. Modulation also increases sensitivity, allowing the detection of weaker transition periods. Test parameters should be set to optimize the machine’s sensitivity for the specific thermal events expected of the material. Adjustments can be made based on which events are being studied. Care should be taken to not exceed the degradation temperature of the material.
Sample preparation[edit | edit source]
The samples are commonly sealed in aluminum pans through a crimping process, to prevent sample and volatile loss. Typical sample masses are between 5-20 mg. Metallic samples are recommended to be around 5 mg while polymeric samples are recommended to be on the higher end around 15 mg. Samples should be kept as flat and thin as possible to maximize the contact surface with the pan, which results in more efficient heat transfer by conduction. A variety of sample pans are available and should be selected depending on the maximum temperature and internal pressure they can withstand. Commonly, hermetic aluminum pans are used. Great care should be taken to prevent any damage to the sample pan as they are very fragile. Damage can affect the quality of heat transfer, leading to inaccurate results.
Analysis of Results[edit | edit source]
A typical DSC graph consists of normalized heat flow on the y-axis and temperature on the x-axis. As the material experiences exothermic and endothermic reactions, transition regions marked by peaks and slope differences appear and can be analyzed using the provided software to characterize the material.
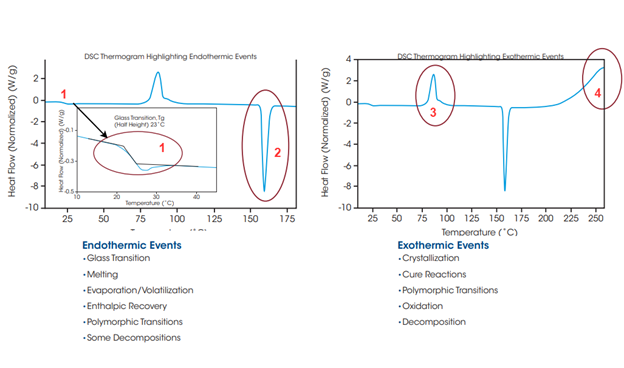
In Figure 2, common thermal events have been marked. Point 1 displays a typical glass transition region without overlapping thermal events. This shows how weak this event can appear. Point 2 displays a typical melting temperature peak. This is marked as a sharp endothermic peak. Point 3 displays a typical crystallization event marked by a sharp exothermic peak. For an example of a cure reaction in a thermoset polymer, please refer to Figure 3 shown below.
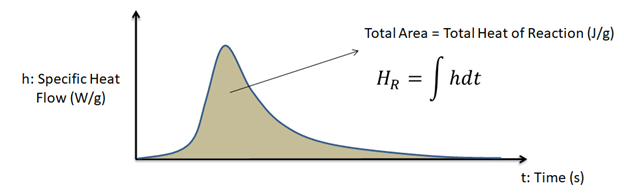
For more information, please see page Heat of reaction.
Reaction Enthalpy[edit | edit source]
The equipment software can be used to integrate over exothermic and endothermic peaks. By selecting the onset points on either end of the peak and choosing to integrate over time, enthalpy can be determined. For thermoset polymers, this feature can be used to determine the enthalpy of cure. For thermoplastic polymers, this can be used to determine the fusion enthalpy.
Glass Transition Temperature (Tg)[edit | edit source]
Although modulated DSC tests are more common for determining Tg, this property can still be determined through regular DSC testing. It is marked by a step change in heat capacity. A region with two linear trends with differing slopes will be observed (see region 1 of Figure 2). The slope of the line connecting these two linear trends provides Tg. This temperature value represents the range over which the glass transition occurs. Care must be taken when analyzing this property as it is affected by the heating and cooling rates in addition to other physical properties.
Special Case 1: Characterization of thermoset polymers via DSC[edit | edit source]
Property of Interest: Degree of Cure[edit | edit source]
DSC provides detailed, reliable data on cure kinetics for thermosetting polymers. Cure kinetics modeling is based on measuring the heat of reaction during cure as a function of various temperatures or heating rates. The exothermic peak is integrated to find the residual heat of reaction. Using the following equation, the DOC can be found.
\( \text{DOC}(t) = 1- \frac{\Delta H(t)}{\Delta H_R} \)
In which ∆H(t) is the measured enthalpy of cure reaction at time t, and ∆H_R is the total enthalpy of cure reaction (fully cured thermoset). This property is useful for quality control purposes and process optimization.
Special case 2: Characterization of thermoplastic polymers via DSC[edit | edit source]
Property of Interest: Crystallization Temperature (Tc)[edit | edit source]
Found from the temperature at the exothermic peak of the cooling cycle in a heat flow (normalized) vs temperature graph. With slower temperature ramps, several peaks may be visible in close proximity to one another. These may represent the formation of different crystal structures. It is worth noting that crystallization is a kinetic process and the formations that occur are greatly affected by the test parameters.
Property of Interest: Melting Temperature (Tm)[edit | edit source]
The sharp endothermic peak on the heating cycle of a normalized heat flow vs temperature graph would be considered the melting temperature of the material. Secondary melting temperatures can be determined using the same method on endothermic peaks occurring on following heat cycles after a cooling cycle.
Additional information on thermal events of polymers can be found here: Thermal phase transitions of polymers.
Combining Equipment[edit | edit source]
Thermogravimetric Analyzer (TGA):[edit | edit source]
It is crucial to always run a TGA test to determine the temperature at which the material degrades before performing DSC testing. Degradation of the sample during testing can cause corrosion of the pan and interfere with results.
Dynamic Mechanical Analyzer (DMA):[edit | edit source]
To provide a more realistic view of a material’s behavior during manufacturing, dynamic testing can be performed to determine Tg and Tm with a dynamic and static load. This results in a lower melting temperature and is a useful property for understanding the material’s limits and true behavior. Performing DSC testing prior to DMA allows one to more efficiently set temperature targets and test parameters.
| About | Help |
Welcome
Welcome to the CKN Knowledge in Practice Centre (KPC). The KPC is a resource for learning and applying scientific knowledge to the practice of composites manufacturing. As you navigate around the KPC, refer back to the information on this right-hand pane as a resource for understanding the intricacies of composites processing and why the KPC is laid out in the way that it is. The following video explains the KPC approach:
Understanding Composites Processing
The Knowledge in Practice Centre (KPC) is centered around a structured method of thinking about composite material manufacturing. From the top down, the heirarchy consists of:
- The factory
- Factory cells and/or the factory layout
- Process steps (embodied in the factory process flow) consisting of:
The way that the material, shape, tooling & consumables and equipment (abbreviated as MSTE) interact with each other during a process step is critical to the outcome of the manufacturing step, and ultimately critical to the quality of the finished part. The interactions between MSTE during a process step can be numerous and complex, but the Knowledge in Practice Centre aims to make you aware of these interactions, understand how one parameter affects another, and understand how to analyze the problem using a systems based approach. Using this approach, the factory can then be developed with a complete understanding and control of all interactions.
Interrelationship of Function, Shape, Material & Process
Design for manufacturing is critical to ensuring the producibility of a part. Trouble arises when it is considered too late or not at all in the design process. Conversely, process design (controlling the interactions between shape, material, tooling & consumables and equipment to achieve a desired outcome) must always consider the shape and material of the part. Ashby has developed and popularized the approach linking design (function) to the choice of material and shape, which influence the process selected and vice versa, as shown below:
Within the Knowledge in Practice Centre the same methodology is applied but the process is more fully defined by also explicitly calling out the equipment and tooling & consumables. Note that in common usage, a process which consists of many steps can be arbitrarily defined by just one step, e.g. "spray-up". Though convenient, this can be misleading.
Workflows
The KPC's Practice and Case Study volumes consist of three types of workflows:
- Development - Analyzing the interactions between MSTE in the process steps to make decisions on processing parameters and understanding how the process steps and factory cells fit within the factory.
- Troubleshooting - Guiding you to possible causes of processing issues affecting either cost, rate or quality and directing you to the most appropriate development workflow to improve the process
- Optimization - An expansion on the development workflows where a larger number of options are considered to achieve the best mixture of cost, rate & quality for your application.
To use this website, you must agree to our Terms and Conditions and Privacy Policy.
By clicking "I Accept" below, you confirm that you have read, understood, and accepted our Terms and Conditions and Privacy Policy.