Polymer (matrix) structure - A236
| Polymer (matrix) structure | |
|---|---|
| Foundational knowledge article | |
| |
| Document Type | Article |
| Document Identifier | 236 |
Overview[edit | edit source]
Polymer materials have molecular structures with long chains comprised of small repeating units. The origin of the word polymer comes from the Greek words poly (many) and meros (part) [1]. Many engineering polymer materials are organic compounds chemically based on carbon, hydrogen, and other non-metallic compounds. Polymers are characteristically low in density and flexible compared to the other material classes [2].
For polymer matrix composites (PMC), both thermoset or thermoset polymers can be used as the matrix. The choice of matrix polymer material determines both the composite’s environmental resistance and maximum service temperature.
Polymer Molecular Structure[edit | edit source]
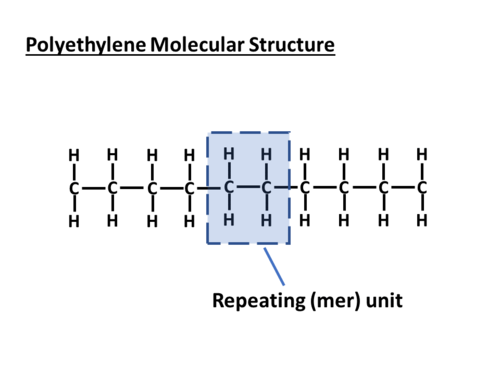
Polymers are comprised of long molecules that are made up of smaller repeating units (also known as a "mer") joined together end to end. Illustrated below is the simple example of polyethylene (PE), with its repeating molecular structure.
As more repeating molecular units are added, the polyethylene molecular chain length grows and extends, and its molecular weight increases (more precisely molecular mass or molar mass – but it is commonly referred to as molecular weight in the polymer literature).
The mechanical properties exhibited by the polymer are dependent on two molecular factors:
- The length of the molecule
- The shape, i.e. configuration, of the molecule
Molecular Length[edit | edit source]
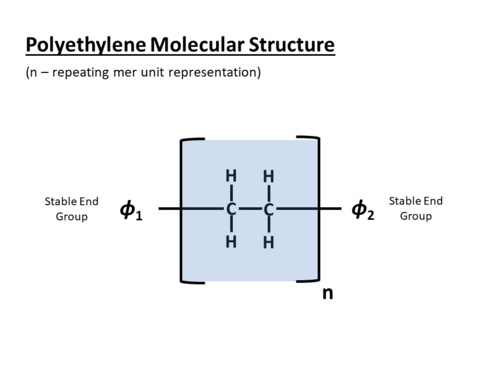
The joining of the repeating molecular units creates a chain-like molecular structure for the polymer. Polymers with very long chains feature extremely large molecular weight [2]. Not all the polymer chains grow to the same length – as a result, the average molecular weight or the average number of repeat units in a chain is typically reported for the polymer.
As the number of repeating units (n) increases, both the physical polymer chain length and the corresponding molecular weight of the polymer increases.
Molecular Configuration[edit | edit source]
There are several molecular configurations that can be observed for polymers. The basic configurations are as follows.
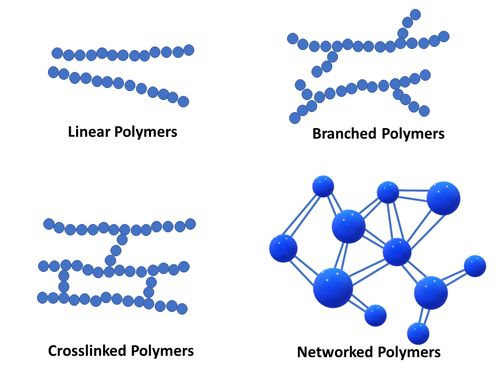
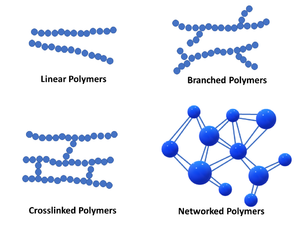
Linear Polymer
Linear polymers consist of long chain-like structures where repeating units are joined together end to end. The long linear chains are flexible, where van der Waals and hydrogen bonding may occur between polymer chains while in close proximity – leading to chain entanglement.
Branched Polymers
Branched polymers feature short side branched chains that are connected to the main (longer) polymer chains. The packing efficiency of the polymer with side branching is reduced, lowering the polymer density.
Crosslinked Polymers
The linear polymer chains of crosslinked polymers are joined together via covalent bonding of smaller molecules acting as bridges between them. The crosslinking process often takes place through a non-reversible chemical reaction process (curing). Crosslink formation characterizes thermoset polymers.
Networked Polymers
Networked polymers feature mer units with three active covalent bonds, and have the ability to form interconnected three-dimensional network configurations [2]. Networked polymers include highly crosslinked polymers. For example, highly crosslinked epoxies are often described to have a highly crosslinked three-dimensional epoxy network configuration.
Microstructure[edit | edit source]
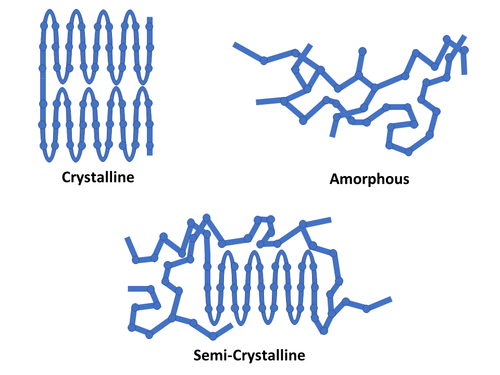
Polymers can exhibit two basic morphologies (structures), or a combination of the two, when “frozen” in their solid state. These basic morphologies are illustrated below.
| Crystalline | Amorphous | Semi-Crystalline |
|---|---|---|
|
|
|
Common observed microstructures[edit | edit source]
Thermoset polymers are generally amorphous in microstructure – a consequence of the lack of chain mobility that results from crosslinking. The polymer chains do not have the physical ability to re-organize into packed and folded molecular chain structures.
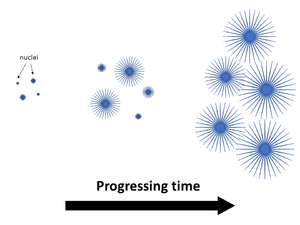
Thermoplastic polymers on the other hand typically exhibit a combination of both amorphous and crystalline regions, forming a semi-crystalline microstructure. The crystalline structures often take the form of spherulites – which form in a polymer that is cooled from its melted molten state back to a solid.
Initially starting as nuclei appearing in a super cooled polymer melt (below melting temperature Tm), the spherulite structures grow outward in the radius direction with progressing time. Spherulite growth is actually the growth of many smaller crystals forming the larger structure [1]. As the spherulites grow, polymer becomes trapped between these structures – the amorphous polymer regions.
Thermoset Polymers[edit | edit source]
Link to main Thermoset polymers page
Thermoset or thermosetting polymers are characterized by the molecular crosslink network of covalent bonds that are formed between adjacent primary polymer chains. Thermosets undergo polymerization and crosslinking during a curing stage with the aid of a hardening agent and heating or promoter.
During curing, they change from viscous fluid to rubbery gel (viscoelastic material) and finally glassy solid. If heated after curing, initially they become soft and rubbery at high temperatures. If further heated, they do not melt but decompose (burn). Thermoset polymers effectively become irreversibly hardened after curing and cannot be reprocessed, making them single use and not recyclable.
Popular examples of thermoset polymers include epoxy and polyester.
Thermoplastic Polymers[edit | edit source]
Link to main Thermoplastic polymers page
A class of polymer that is characterized to have the ability to flow when heated. Typically linear or branched in structure, upon heating, thermoplastics soften and melt where they flow in a manner of a viscous liquid. This process is repeatable upon repeated heating and cooling, making them potentially recyclable.
The high viscosity of thermoplastics when melted, make it difficult to saturate fibers in the composite manufacturing process. A lot of pressure and heat are required to process.
Some common examples of popular thermoplastics include polypropylene and polyethylene.
Explore this area further
References
- ↑ 1.0 1.1 [Ref] McCrum, N. G. et al. (1997). Principles of Polymer Engineering. Oxford University Press. ISBN 978-0-198565-26-0.CS1 maint: extra punctuation (link) CS1 maint: uses authors parameter (link) CS1 maint: date and year (link)
- ↑ 2.0 2.1 2.2 [Ref] Callister, William D. (2003). Materials Science and Engineering: An Introduction. John Wiley & Sons, Inc. ISBN 0-471-13576-3.CS1 maint: uses authors parameter (link) CS1 maint: date and year (link)
| About | Help |
Welcome
Welcome to the CKN Knowledge in Practice Centre (KPC). The KPC is a resource for learning and applying scientific knowledge to the practice of composites manufacturing. As you navigate around the KPC, refer back to the information on this right-hand pane as a resource for understanding the intricacies of composites processing and why the KPC is laid out in the way that it is. The following video explains the KPC approach:
Understanding Composites Processing
The Knowledge in Practice Centre (KPC) is centered around a structured method of thinking about composite material manufacturing. From the top down, the heirarchy consists of:
- The factory
- Factory cells and/or the factory layout
- Process steps (embodied in the factory process flow) consisting of:
The way that the material, shape, tooling & consumables and equipment (abbreviated as MSTE) interact with each other during a process step is critical to the outcome of the manufacturing step, and ultimately critical to the quality of the finished part. The interactions between MSTE during a process step can be numerous and complex, but the Knowledge in Practice Centre aims to make you aware of these interactions, understand how one parameter affects another, and understand how to analyze the problem using a systems based approach. Using this approach, the factory can then be developed with a complete understanding and control of all interactions.
Interrelationship of Function, Shape, Material & Process
Design for manufacturing is critical to ensuring the producibility of a part. Trouble arises when it is considered too late or not at all in the design process. Conversely, process design (controlling the interactions between shape, material, tooling & consumables and equipment to achieve a desired outcome) must always consider the shape and material of the part. Ashby has developed and popularized the approach linking design (function) to the choice of material and shape, which influence the process selected and vice versa, as shown below:
Within the Knowledge in Practice Centre the same methodology is applied but the process is more fully defined by also explicitly calling out the equipment and tooling & consumables. Note that in common usage, a process which consists of many steps can be arbitrarily defined by just one step, e.g. "spray-up". Though convenient, this can be misleading.
Workflows
The KPC's Practice and Case Study volumes consist of three types of workflows:
- Development - Analyzing the interactions between MSTE in the process steps to make decisions on processing parameters and understanding how the process steps and factory cells fit within the factory.
- Troubleshooting - Guiding you to possible causes of processing issues affecting either cost, rate or quality and directing you to the most appropriate development workflow to improve the process
- Optimization - An expansion on the development workflows where a larger number of options are considered to achieve the best mixture of cost, rate & quality for your application.
To use this website, you must agree to our Terms and Conditions and Privacy Policy.
By clicking "I Accept" below, you confirm that you have read, understood, and accepted our Terms and Conditions and Privacy Policy.